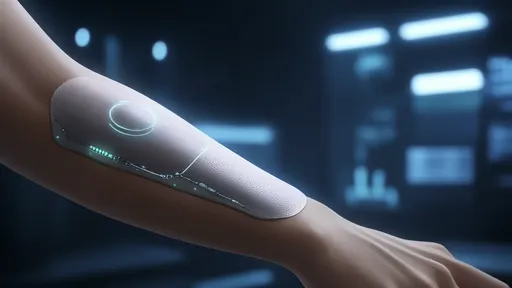
The field of medical diagnostics is witnessing a revolutionary shift with the advent of nanotechnology, particularly in the detection of lung cancer biomarkers. Traditional methods for diagnosing lung cancer often involve invasive procedures, delayed results, and limited sensitivity. However, the integration of nanosensors into respiratory diagnostic devices is paving the way for non-invasive, real-time, and highly accurate detection of lung cancer markers. This breakthrough promises to transform early diagnosis and improve patient outcomes.
Nanotechnology has unlocked new possibilities in biomarker detection by leveraging the unique properties of nanomaterials. These materials, such as gold nanoparticles, carbon nanotubes, and quantum dots, exhibit exceptional electrical, optical, and chemical characteristics. When functionalized with specific biorecognition elements, they can selectively bind to lung cancer biomarkers in exhaled breath, such as volatile organic compounds (VOCs) or specific proteins. This binding event generates measurable signals, enabling precise and rapid diagnosis.
One of the most compelling advantages of nanosensor-based respiratory diagnostics is its non-invasive nature. Unlike biopsies or blood tests, which can be uncomfortable and time-consuming, breath analysis offers a painless and convenient alternative. Patients simply exhale into a device equipped with nanosensors, which then analyze the breath for the presence of cancer-related biomarkers. This approach not only enhances patient compliance but also allows for frequent monitoring, which is critical for early detection and treatment efficacy.
The sensitivity and specificity of nanosensors far surpass conventional diagnostic techniques. For instance, some nanosensors can detect biomarkers at concentrations as low as parts per billion, making them ideal for identifying early-stage lung cancer when biomarker levels are minimal. Additionally, the ability to multiplex—detecting multiple biomarkers simultaneously—further increases diagnostic accuracy. This is particularly important for lung cancer, which often presents with heterogeneous biomarker profiles.
Despite these advancements, challenges remain in the widespread adoption of nanosensor-based respiratory diagnostics. Standardization of sensor fabrication, reproducibility of results, and integration with existing healthcare systems are key hurdles that researchers are actively addressing. Moreover, the cost of nanomaterials and the need for specialized equipment may limit accessibility in resource-limited settings. However, ongoing innovations in scalable manufacturing and portable devices are expected to mitigate these barriers.
Looking ahead, the convergence of nanotechnology, artificial intelligence, and wearable devices holds immense potential for respiratory diagnostics. AI algorithms can analyze vast datasets generated by nanosensors to identify subtle patterns and improve diagnostic accuracy. Wearable breath analyzers, equipped with nanosensors, could enable continuous monitoring of at-risk individuals, providing real-time alerts for early intervention. This holistic approach could redefine lung cancer management, shifting the paradigm from reactive treatment to proactive prevention.
The implications of this technology extend beyond lung cancer. The principles of nanosensor-based breath analysis can be adapted for other diseases, such as asthma, chronic obstructive pulmonary disease (COPD), and even infectious diseases like tuberculosis. As research progresses, the scope of respiratory diagnostics will likely expand, offering a versatile tool for global health challenges. The marriage of nanotechnology and medical diagnostics is not just a scientific achievement—it is a lifeline for millions of patients worldwide.
By /Jul 18, 2025

By /Jul 18, 2025

By /Jul 18, 2025
By /Jul 18, 2025
By /Jul 18, 2025
By /Jul 18, 2025
By /Jul 18, 2025
By /Jul 18, 2025

By /Jul 18, 2025

By /Jul 18, 2025

By /Jul 18, 2025

By /Jul 18, 2025

By /Jul 18, 2025

By /Jul 18, 2025

By /Jul 18, 2025

By /Jul 18, 2025

By /Jul 18, 2025

By /Jul 18, 2025
By /Jul 18, 2025
By /Jul 18, 2025